
The project started on the 1st of January 2019 and will run until the 31st of December 2021.
Consortium
Coordinated by ERINHA AISBL (Belgium) with the support of Inserm Transfert (France), the consortium brings together 11 partners from 8 European countries (Belgium, France, Portugal, Sweden, Hungary, Italy, Austria and Germany). 5 of these 8 European countries possess national BSL4 facilities (Inserm France, FoHM Sweden, NPHC Hungary, INMI Italy, BNI Germany), 4 of them (KUL Belgium, INSA Portugal, KI Sweden, MUG Austria) have complementary facilities essential for a research infrastructure dedicated to the study of high-consequence pathogens of Risk Group 4 (RG4). 4 of the 5 ERINHA AISBL members belong to the ERINHA-Advance consortium (Inserm, INSA, FoHM and NPHC).
 |  |  |  |
 |  |  |  |
 |  |  |
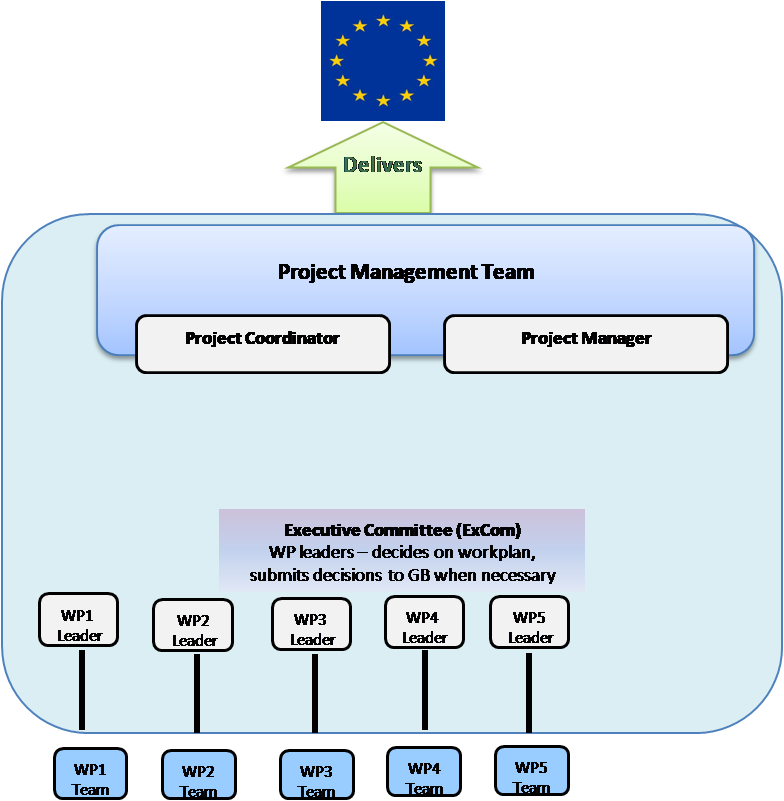
Project Coordination
The project is coordinated by Hervé Raoul, Director General of the ERINHA RI with the support of the infrastructure’s Central Coordinating Unit (CCU) and Inserm Transfert which forms the Project Management Team.

Hervé Raoul, Chair of the Executive Board & coordinator of ERINHA-Advance.
The Executive Committee, gathers the WorkPackage leaders and ensures that the project runs smoothly. It also submits any important decisions to the Governing Board for approval when necessary. The Governing Board is made up of one representative from each consortium member and is the ultimate decision‑making body of the project.
ERINHA-Advance advisory boards
The boards set up by the ERINHA Research Infrastructure will also advise ERINHA-Advance on the project progress: an Independent Advisory Board and a Board of Industrial Representatives.
Activities
The ERINHA-Advance project is organized into 7 Workpackages – working groups dedicated to a specific set of activities.
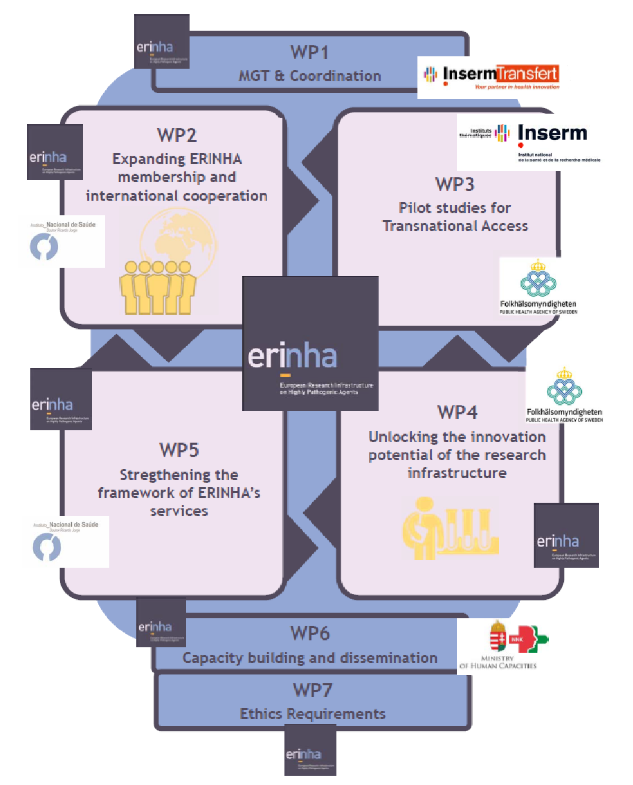
WP1 (ERINHA/IT) aims to manage the project, including communication with the European Commission, financial management, preparation of periodic progress and financial reports.
WP2 (ERINHA/NKK) aims to increase the membership of the Research Infrastructure within the EU and to further develop international links outside Europe in order to reinforce the sustainability of ERINHA and increase Europe’s preparedness level if faced with an epidemic involving RG4 or unknown pathogens.
WP3 (INSERM/FoHM) aims to improve ERINHA AISBL users’ services through the implementation of excellence-driven pilot calls for access to ERINHA facilities. Two programmes will be developed: one on the development of innovative animal models for highly infectious agents and another on an innovative therapeutic approach for RG4 pathogens. The success of this WP will feed into WP2 and WP4.
WP4 (FoHM/ERINHA) aims to stimulate and promote the use of ERINHA services by industrial partners and SMEs in order to facilitate the interaction with European industrial and commercial partners and to identify the opportunities of co-innovation for developing equipment/technologies for ERINHA member facilities.
WP5 (ERINHA/INSA) aims to ensure the sustainability and effectiveness of high quality ERINHA services provided to private and public entities by implementing a quality management system, an effective IPR and ethical framework and a comprehensive data management and policy plan. The success of this WP will contribute to WP2, WP3 and WP4.
WP6 (ERINHA/NKK) aims to reinforce the capacity building of the Human Resources of ERINHA, and to communicate and disseminate the progress and results of all WPs.
WP7 aims to ensure compliance with the ethics requirements set out for this WP.
The current pandemic of COVID-19 is unprecedented in modern times, and has caused a global crisis with considerable health, economic and social consequences. With no approved medical countermeasures available to fight SARS-CoV-2, it has also ignited a major scientific momentum to better understand this new virus, limit its spread and eventually stop the pandemic.
ERINHA – the European Research Infrastructure on Highly pathogenic Agents – and its Members have come forward in this time of emergency and taken part in multiple European projects and initiatives to advance COVID-19 / SARS-CoV-2 research and accelerate the development of therapeutic and prophylactic solutions against the virus.
However, ERINHA was not only created to lead excellence driven research but also to benefit the whole scientific community. One of the most important missions of the infrastructure is to give academia and industry the opportunity to access Europe’s top high containment research facilities to develop medical countermeasures against highly pathogenic viruses.
Therefore, we now want to extend our commitment to the current scientific effort by launching a call for proposals fully dedicated to COVID-19 / SARS-CoV-2 research. With the support of the EU funded H2020 project ERINHA-Advance, we will be offering the selected scientists and their projects free of charge* transnational access (TNA) to our cutting edge high containment facilities.
* Free of charge transnational access includes administrative & logistical support, free use of the installations, in accordance with all applicable national laws, local safety and health regulations, and technical & scientific support. The User Group will be in charge of procuring consumables & animals (when applicable). Routine consumables may be covered to some extent. These aspects will be discussed during project implementation.
Please note that due to all current travel restrictions, we will not be able to offer training or in person access in the frame of this call.
The selection of the projects will be based on peer review & scientific merit through fast track evaluation. In addition to scientific excellence, priority will be given to:
In addition, applications must fall into one of the following scientific sections listed below. Applications that require both in vitro and in vivo work are welcome.
Proposals in this Section may include:
Proposals in this Section may include:
Proposals in this Section may include:
Proposals in this Section may include any COVID-19 / SARS-CoV-2 related project progressing into GMP production and aiming to set standard operating procedures for production of plasmids, cells, gene modified cells, or viruses; etc.
Proposals in this Section may include:
For any question, please contact our staff at the Central Coordinating Unit (contact@erinha.eu).
To apply, please download the ERINHA-Advance TNA Programme Guidelines, the Application Form and the Data Protection Policy (one form must be signed by each User listed in a User Group).
Please make sure that you accurately describe the methodology related to your Project. This is crucial for us to estimate that we have appropriate capacities to conduct the work. Your Proposal may otherwise not pass the Feasibility Assessment.
An organization can submit several applications.
Opening of the call: Monday 25 May, 2020
Submission deadline: Proposals are recommended to be submitted prior to Monday 22 June, 2020 for priority access. Proposals received after 22 June will be evaluated, selected and implemented on a rolling basis until TNA budget is consumed.