The ISIDORe project assembles the largest and most diverse research and service-providing instrument to study infectious diseases in Europe, with expertise from structural biology to clinical trials.
By giving scientists access to the whole extent of our state-of-the-art facilities, cutting-edge services, advanced equipment, and expertise, in an integrated way and with a common goal, we will accelerate the generation of new knowledge and intervention tools to enhance Europe’s capacity for controlling (re)emerging and epidemic infectious diseases, starting with the COVID-19 pandemic.
Under the umbrella of 17 major European life sciences research infrastructures and infectious diseases networks coordinated by ERINHA, ISIDORe bring together 154 research entities and organizations providing services to advance research on epidemic-prone diseases.
ISIDORe supports scientists and their research on epidemic- and pandemic-prone pathogens, the development of medical countermeasures, with the aim of increasing resilience in the face of epidemics in Europe and globally. It provides free transnational access to a comprehensive portfolio of high-quality services that support user projects. See the full catalogue of services here.
The provision of research services to researchers is organised through calls for proposals and the selection of research projects to access ISIDORe resources for free. Learn about Transnational Access and see the open calls for proposals.
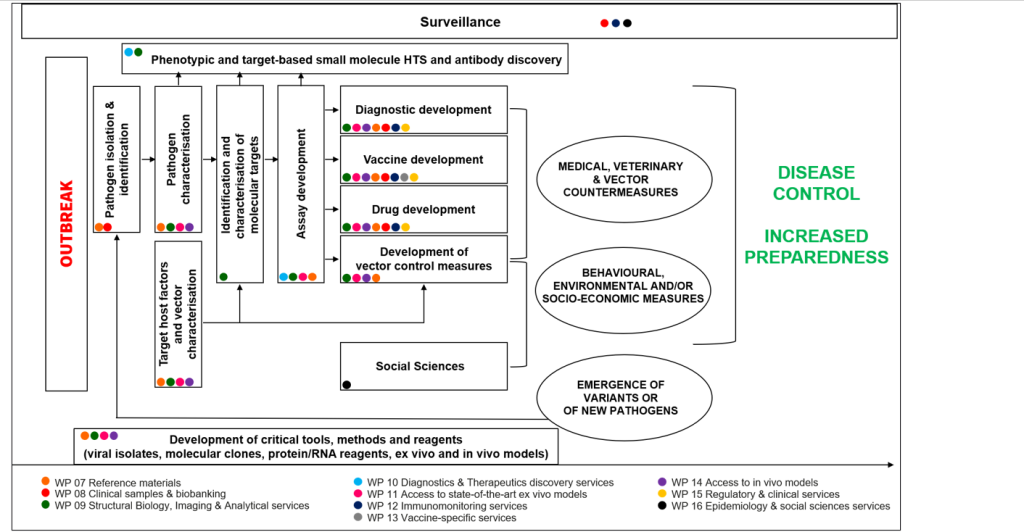
ISIDORe is a unique European integrated large-scale research infrastructure capacity for conducting research on any epidemic-prone pathogen, including SARS-CoV-2. It will be a powerful instrument at the disposal of the EU, and will impact its capacity to identify, characterise and mitigate the effects of any epidemic-prone pathogens.
ISIDORe aims to enable ground-breaking discoveries and the development of new medical countermeasures with high impact on health security and society.
Download the ISIDORe application form
Consortium
Our mission here is to increase EU’s responsiveness and preparedness to any epidemic threats by providing an impressive range of innovative, high-quality services to all scientific User communities with projects in the field of high-consequence pathogens. The ISIDORe consortium represents the biggest research instrument dedicated to the study of high consequence pathogens ever set up.
Following the HERA’s incubator call for expressions of interest on SARS CoV_2 variants, the ERINHA-led project ISIDORe (Integrated Services for Infectious Disease Outbreak Research) was selected as one of the first Horizon Europe actions. The ISIDORe consortium includes 154 participants from 32 countries worldwide and will contribute to research efforts on the SARS-CoV-2 variants and any other epidemic-prone pathogen, by assembling an integrated and complementary portfolio of state-of-the-art, cutting-edge services for the benefit of the scientific community.
.
 |  |  |  |
 | EMERGEN |  |  |
 |  |  |  |
 |  |  |  |
 |
Project Coordination
ERINHA is the Coordinator of the ISIDORe project.
ERINHA and Inserm Transfert form the Project Management Team, responsible for the monitoring and administration of the project.
The Governing Board (GB) is the ultimate decision-making body of the Consortium; it will be in charge of the strategic evaluation of the project’s progress, decide on the overall development of the project, take the necessary actions and corrective measures should the need arise, etc. The GB is made up of one representative from each Research Infrastructure and Network of the core consortium. These 17 Ris and networks act on behalf of their access providers in the project.
The Executive Committee (ExCom) will be the supervisory body for the execution of the action and implementation of the decisions taken by the GB. The ExCom will perform the strategic scientific steering of the project and is made up of the Work Package (WP) leaders and deputy-leaders.
ISIDORe will implement a multi-purpose Independent Advisory Board (IAB) with four distinct sections, which will be made up of the appropriate experts to support the project in strategy, science, ethics, security. ISIDORe IAB will act as an interface with the relevant international agencies, initiatives, projects and individual stakeholders.
The four sections of the IAB are:
• The Strategic Advisory Board (StAB) will be consulted to define the scope of ISIDORe’s TNA programmes and of the topics of the calls for proposals that will be launched. The goal is to align ISIDORe’s activities with the priorities of actors of pandemic management and preparedness, funders, regulatory decision-makers, the pharmaceutical industry, etc. StAB members will include representatives from the most relevant agencies and initiatives, such as HERA, ECDC, WHO, CEPI, GloPID-R, OIE, FAO, EMA, and EFPIA.
• The Scientific Advisory Board (SciAB) will make recommendations on major trends and priorities in the field of epidemic-prone pathogens. The ScIAB will be made up of individuals who are experts in infectious diseases from the academic and private sectors, coordinators of major infectious disease-related actions and any other relevant expert.
• The Ethics Advisory Board (EthAB) will include a minimum of three experts – one in clinical studies, one in animal welfare, and one in data protection – who will monitor ISIDORe’s compliance with all relevant ethical regulations and standards pertaining to the activities of the project.
• The Security Advisory Board (SecAB) will to review the project deliverables, evaluate the sensitivity of the information that they contain and, if necessary, propose timely measures for preventing the misuse of such information. The SecAB will be composed of five (5) members, including vetted representatives of biology research organisations and accredited virologists with competence and experience of the issues raised by the above security concerns.
Activities
The ISIDORe project is organized into 17 Workpackages – working groups dedicated to a specific set of activities.
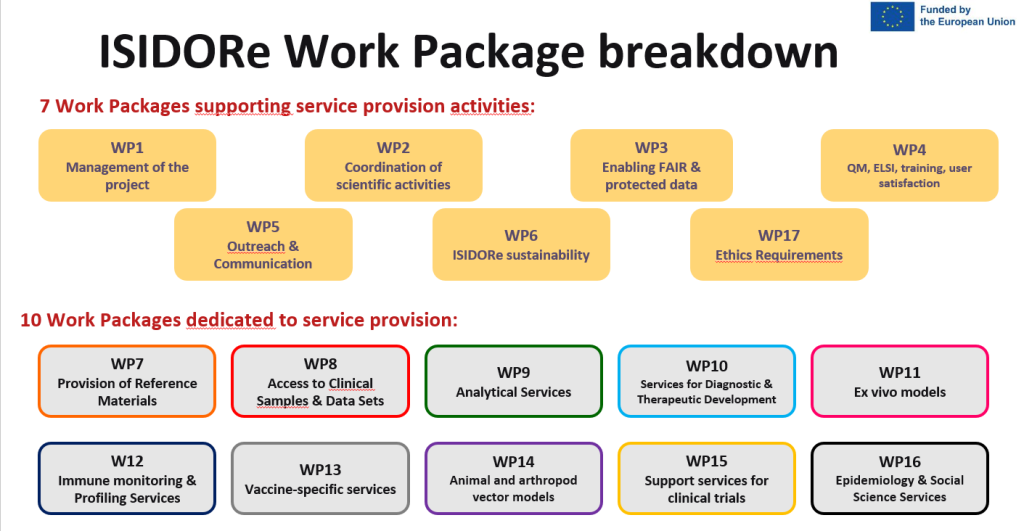
The objective is to provide efficient management and coordination of the project and the consortium and to ensure the progress of the project towards its planned objectives.
The objective of WP2 is to coordinate all the scientific activities of ISIDORe and to coordinate the Joint Research Activities (JRAs) that will be necessary to expand the service catalogue in accordance with the evolving needs of the scientific communities, starting with service provision through transnational access. Selected individual researchers or teams (users), from academia or the private sector, will benefit from our capacities and services through the provision of “in-person”, remote and virtual transnational access to support their research on epidemic-prone pathogens.
This WP will particularly focus on FAIR data management of all ISIDORe data, define and make ISIDORe research data emerging from access provision activities available, reuse common data platforms and registries, according to FAIR principles and the General Data Protection Regulation.
This work package takes on the task of holistically collecting applicable quality, safety, ethical and regulatory requirement, developing a standardised approach for obtaining structured feedback from ISIDORe service providers and users and developing an ISIDORe training and education concept.
This work package will be focusing on providing a world-class engagement approach and supporting networks, creating a project-specific collaboration plan and the channels of communication, determining which communities could be interested in using the services and associated data, displaying the value of transparent data and services, and providing public and community outreach, training, dissemination material and feedback about services.
WP6 will develop a concrete pathway towards ISIDORe sustainability in the long run. This will be achieved by two main approaches: i) a stakeholder engagement strategy, to collect input from stakeholders and increase visibility of ISIDORe within the One Health field, and ii) development and implementation of a Sustainability Plan for the ISIDORe entity, taking in account the heterogeneity of the mandates, size and business models among the 17 partners.
WP7 gathers the all the activities dedicated to the selection, development, production, characterization, and qualification of infectious and non-infectious material necessary for the services in the scope of ISIDORe.
This work package is in charge of providing retrospective and on demand collections of high-quality human biological samples (for the purpose of research projects on COVID-19 and different SARS-CoV-2 variants as well as epidemic prone pathogens in general) and providing high-quality associated data.
This WP will be oriented to identify and provide advice on the roles of structural biology, imaging and other analytical services to address scientific questions at various checkpoints along the rapid response pipeline to combat an infectious disease epidemic and to provide structural biology services; imaging services; analytical services.
P10 main objective is to provide orthogonal and relevant drug discovery services required by user demands to respond with new therapeutics to SARS-CoV-2 variants and epidemic prone pathogens in general. Candidates for therapeutic development are small molecule anti-viral modulators, antibacterial and anti-inflammatory/immunomodulatory compounds, and neutralizing/blocking antibodies.
The objective of WP11 is to support users in conducting ex vivo scientific projects related to SARS-CoV2 and its variants, as well as other epidemic prone pathogens. sing relevant and standardized cellular or tissue-based ex vivo systems allow the establishment of robust proofs of concept while reducing the number of in vivo studies according to the 3R principles.
WP12 will provide high-quality services that will speed up drug and vaccine development process by offering the users a wide range of state-of-the-art immune assays for clinical and translational studies based on the highest standard operating systems from feasibility to validation through potential optimization. Access to immunomonitoring services will be provided by multiple institutions with vast expertise and capacity to cutting-edge technological services from six major infrastructures within the consortium.
This WP aims at providing services that are specifically needed in the field of vaccine development (GLP and GMP production, access to adjuvants and formulation services (GLP and GMP), etc.).
Animal studies are indispensable, and in certain cases mandatory, to study infectious processes or to test and/or validate novel therapies, drugs and vaccines. The conduct of such studies requires expertise, capacities and technical facilities that are scarce, notably when it comes to works with RG3-4 pathogens. Similarly, research into the transmission and control of arthropod-borne pathogens requires scientific expertise, technical know-how and rare bio contained insectaries and facilities.
WP15 will support interventions reaching the clinical development step as they represent a key component of the infrastructure support to the development or repurposing of
treatment or vaccine interventions.
WP16 will provide a complete catalogue of services necessary to investigate and act on the range of phenomena from pathogen emergence to epidemic outbreak and societal response. We offer available services to study across the range of systems spanning natural field observatories and human societies, with integrated data services, risk mapping, and community surveillance. We also include services to characterize nucleotide differences between SARS-CoV-2 variants to provide actionable data in near real-time.
The objective is to ensure compliance with the ‘ethics requirements’ set out in this work package.
What is a transnational access (TNA) ?
Free of charge Transnational Access to research facilities, services, resources and tools. ISIDORe TNA programme aims at supporting scientists by providing them access (physical, remote, or virtual) to facilities, equipment, expertise, services and resources that they do not usually have access to.
There are three ways for scientists of benefiting from TNA:
The access is free of charge and includes the logistical, technological and scientific support and, when appropriate, the specific training that is needed to use the services provided by the research facilities.
Eligibility
The ISIDORe project is a very new type of action that is almost entirely dedicated to providing support to scientific communities. To do so as fast as possible, we are currently relying on temporary application modalities and procedures that will be improved as ISIDORe unfolds. Please bear with us as we work on making this happen, and do not hesitate to contact us for any questions.
Proposals for accessing any of the services of the ISIDORe partner facilities are eligible if they meet the following criteria:
How to apply?
If you wish to benefit from the support of ISIDORe, you are requested to submit proposals that will be evaluated based on their feasibility and scientific merit (independent peer review).
Three steps to get free-of-charge access to our services:
Before you navigate our catalogue, please remember that for a pre-application submission: